- Radiographic progression-free survival

Your patients with metastatic castration-resistant prostate cancer (mCRPC) are invited to join ECLIPSE
Participating in this 177Lu-PSMA-I&T trial presents an investigational therapy option for your patients that may help advance innovation in mCRPC treatment
Hear more about this ground-breaking trial
In this 15-minute podcast, Sakir Mutevelic, MD, MSc, Curium’s chief medical officer, discusses the ECLIPSE trial in greater detail, including eligibility criteria and how to enroll patients. In addition, he describes the role of radiopharmaceuticals in diagnosing and treating prostate cancer and a patient’s typical diagnostic journey.
 Targeting PSMA can initiate cell death
Targeting PSMA can initiate cell death
PSMA (prostate-specific membrane antigen) is an attractive target because it is highly expressed on the cell surface of prostate cancer tumors1
When 177Lu-PSMA-I&T binds to the extracellular portion of PSMA, it is taken into the cell, where the radiation causes DNA damage to initiate cell death2
See 177Lu-PSMA-I&T in action
Learn more about dosing administration for 177Lu‑PSMA‑I&T
 Purpose
Purpose
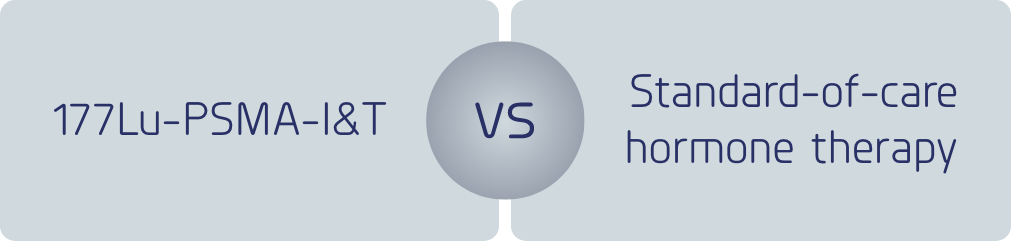
The purpose of this study is to compare the safety and efficacy of 177Lu-PSMA-I&T versus standard-of-care hormone therapy in patients with metastatic castration-resistant prostate cancer.
 Study design
Study design
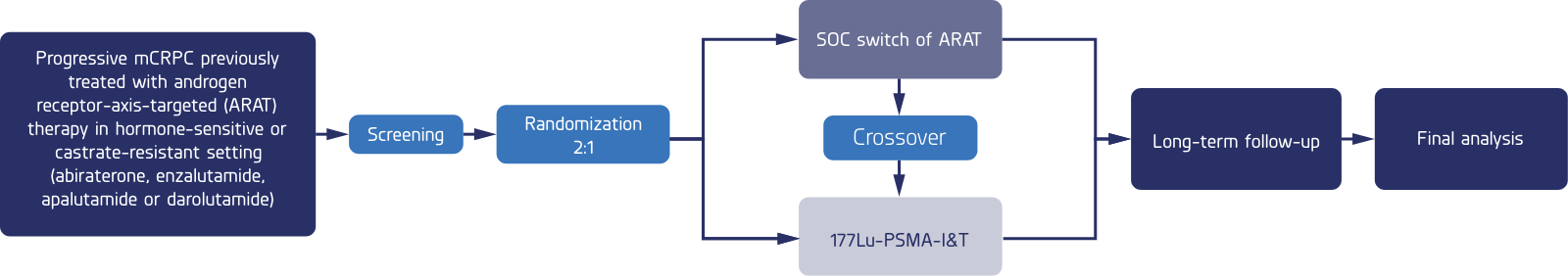
A multicenter, open-label, randomized phase 3 trial comparing the safety and efficacy of 177Lu-PSMA-I&T versus hormone therapy in patients with metastatic castration-resistant prostate cancer, previously treated with only one ARAT and are taxane-based chemonaive.
Click below to expand content
- Overall survival
- Second radiographic progression
- Progression-free survival (PFS, composite)
- Progression-free survival 2 (PFS2, composite)
- PSA50 response rate
- Impact on skeletal symptoms
- Change in use of chemotherapy
- Quality of life
- Safety
- Objective response rate
- Disease control rate
- Time to PSA progression
- Duration of response
- Pharmacokinetics and dosimetry

Patients will be randomized using a 2:1 ratio of study medication to standard of care
This trial is a crossover study in which those patients randomized to the standard-of-care arm may have the option to receive the study drug if disease progression is detected while on standard-of-care hormone therapy.
 The typical patient journey and the potential
path to 177Lu‑PSMA‑I&T therapy
The typical patient journey and the potential
path to 177Lu‑PSMA‑I&T therapy

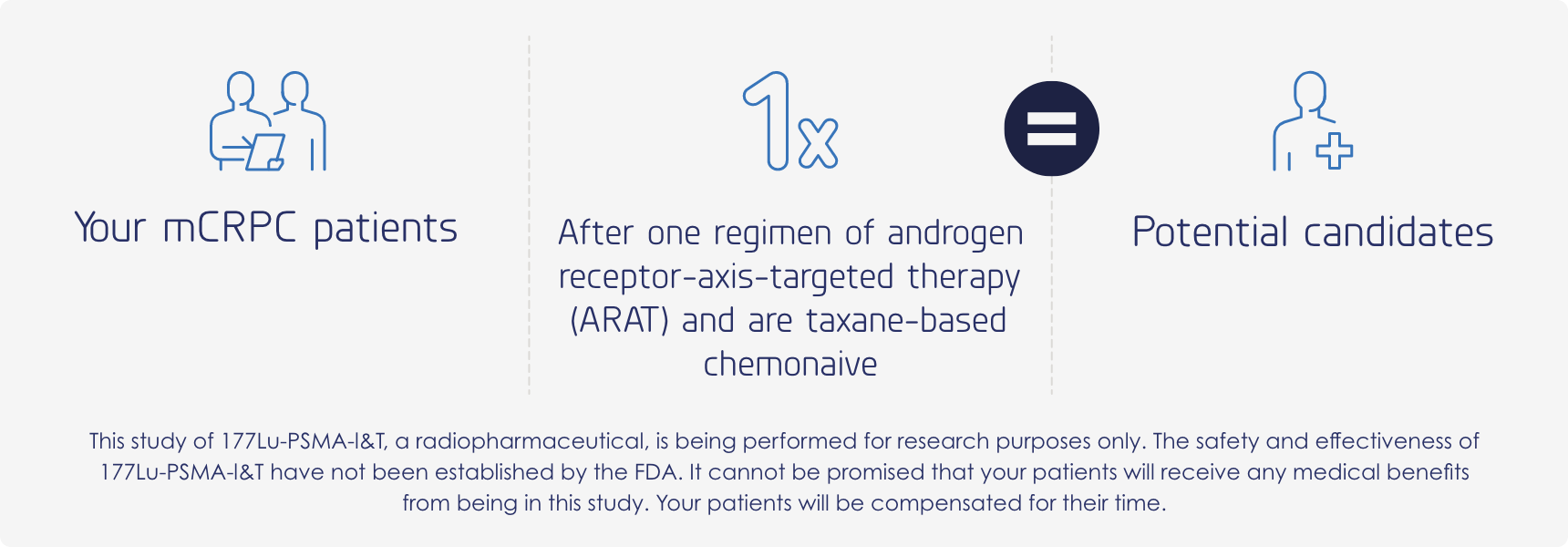
Patients may follow similar paths, yet every treatment journey is different. Your patients may qualify for this trial; however, specific requirements will need to be met.
Major inclusion criteria
- Males, 18 years or older
- Proven metastatic castration-resistant prostate cancer
- Progressive disease while on androgen receptor-axis-targeted (ARAT) therapy (abiraterone, enzalutamide, darolutamide, apalutamide)
- Positive PSMA PET scan
Major exclusion criteria
- Previous treatment with more than one ARAT
- Previous treatment with chemotherapy in the castration-resistant setting
- Previous treatment with other radioligand therapy
Contact eclipse@curiumpharma.com for a full list of inclusion and exclusion criteria
 Directory of ECLIPSE trial sites across the United States
Directory of ECLIPSE trial sites across the United States
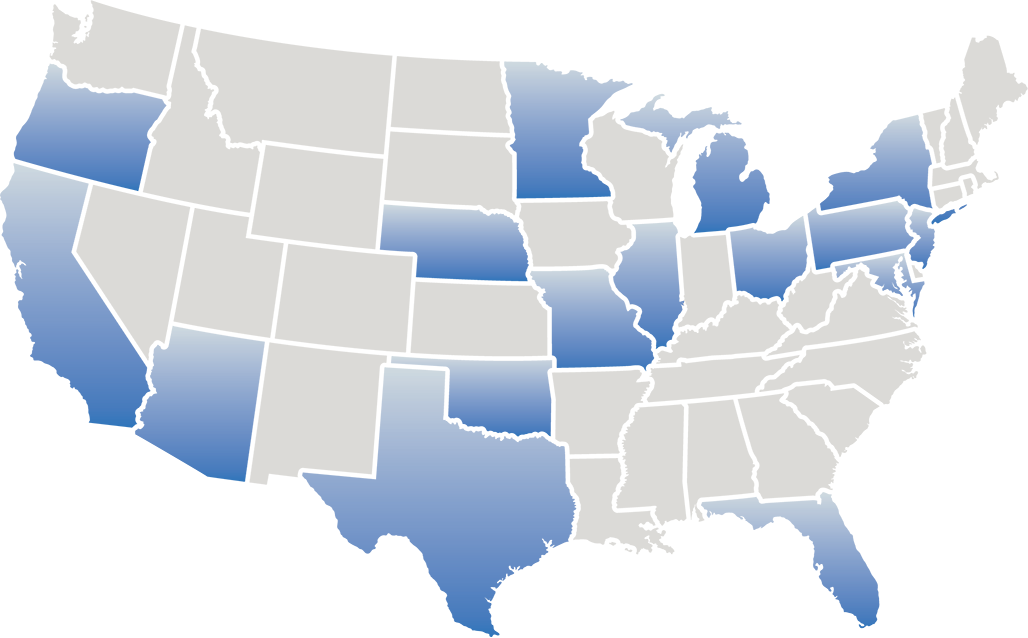
Please contact these sites directly for more information about eligibility
Click on a state below to see trial sites and primary investigators
-
Arizona Institute of Urology (AIU) - Tucson
Kalpesh Patel, MD
-
Saint John’s Cancer Institute - Santa Monica
Przemyslaw Twardowski, MD
-
Providence Medical Foundation - Fullerton
Sanjay Sharma, MD
-
Hoag Family Cancer Institute - Laguna Beach
Gary Ulaner, MD
-
University of California San Francisco (UCSF) Medical Center
Vadim Koshkin, MD
-
San Francisco VA
Franklin Huang, MD, PhD
-
MemorialCare - Long Beach
Amol Rao, MD
-
Biogenix Molecular - Miami
Frankis Almaguel, MD, PhD
-
GenesisCare USA - Boca Raton
Vinay Sharma, MD
-
GenesisCare USA - Plantation
Christopher Chen, MD
-
Florida Urology Partners - Tampa
Alexander Engelman, MD
-
Orlando Health - Orlando
Daniel Landau, MD
-
Northwestern Memorial - Chicago
David James VanderWeele, MD, PhD
-
NorthShore Kellogg Cancer Center - Evanston
Daniel Shevrin, MD
-
Johns Hopkins
Steven Rowe, MD, PhD
-
Mid-Atlantic Permanente Research Institute - Gaithersburg
Philipose Mulugeta, MD
-
GenesisCare USA - Troy
Tom Boike, MD
-
BAMF Health - Grand Rapids
Brandon Mancini, MD, MBA, FACRO
-
Henry Ford Health System
Clara Hwang, MD
-
Michigan Institute of Urology (MIU) - Troy
Jason Hafron, MD
-
M Health Fairview
Gautam Jha, MD
-
Saint Louis University School of Medicine
Razi Muzaffar, DO
-
Nebraska Cancer Specialists
Ralph Hauke, MD, FACP
-
GU Research Network - Omaha
Luke Nordquist, MD, FACP
-
Rutgers University Medical Center - New Brunswick
Tina Mayer, MD
-
Columbia University Medical Center/Herbert Irving Pavilion
Mark Stein, MD
-
Mt Sinai Hospital
Munir Ghesani, MD
-
SUNY Upstate - Syracuse
Gennady Bratslavsky, MD
-
Central Ohio Urology - Columbus
Benjamin Martin, MD
-
The Urology Group - Cincinnati
Marc Pilskin, DO
-
SSM Health Oklahoma City (Hightower Clinical) - Oklahoma City
David Lam, MD
-
Oregon Health & Science University (OHSU)
Jacqueline Vuky, MD
-
VA Portland
Julie Graff, MD
-
MidLantic Urology - Bala Cynwyd
Laurence Belkoff, DO, MSc, FACOS
-
Urology San Antonio
Daniel Saltzstein, MD
contact us
 Get answers to questions about ECLIPSE and patient eligibility
Get answers to questions about ECLIPSE and patient eligibility
References: 1. Hupe MC, Philippi C, Roth D, et al. Expression of prostate-specific membrane antigen (PSMA) on biopsies is an independent risk stratifier of prostate cancer patients at time of initial diagnosis. Front Oncol. 2018;8:623. doi:10.3389/fonc.2018.00623 2. Ruigrok EAM, van Vliet N, Dalm SU, et al. Extensive preclinical evaluation of lutetium-177-labeled PSMA-specific tracers for prostate cancer radionuclide therapy. Eur J Nucl Med Mol Imaging. 2021;48(5):1339-1350. doi:10.1007/s00259-020-05057-6